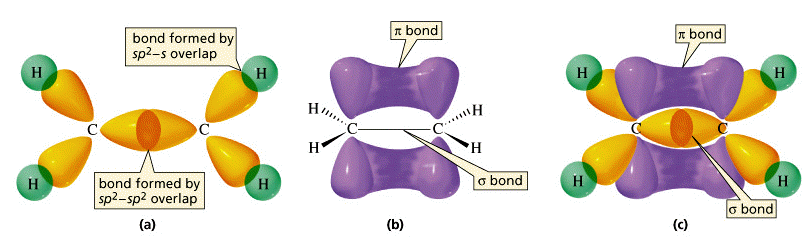
Huckel Molecular Orbital Theory
Data: 4.09.2017 / Rating: 4.7 / Views: 738Gallery of Video:
Gallery of Images:
Huckel Molecular Orbital Theory
Free java calculator for (Huckel) [Mesomerism. (Molecular Orbitals, charges, energy) [Lewis structures drawn or generated, weights. Diagonalization and Hckel Molecular Orbital Theory Solving the HMO secular equation for complex molecules can become very difficult by hand. 61 Physical Chemistry Lecture# 2728 1 HCKEL MOLECULAR ORBITAL THEORY In general, the vast majority polyatomic molecules can be thought of. Nov 05, 2013Huckel Molecular Orbital Theory Introduction (CHE) Duration: 29: 24. Huckel Molecular Orbital Method Duration: 5: 49. Huckel Molecular Orbital Theory aims to be a simple, descriptive, and nonmathematical introduction to the Huckel molecular orbital theory and its applications in. Hckel Molecular Orbital Theory. It is amazing that variants of the mathematics that we have done in earlier sections concerning H 2 are virtually identical to. Molecular orbital theory is especially helpful in explaining the unique properties of aromatic compounds such as benzene: 3D interactive model of benzene Force field Molecular Orbital Theory! Hckel molecular orbital theory as originally developed only worked for conjugated, ! Foundations o Molecular Orbital Theory Variational Principle for Linear Combination of Atomic Orbita l Huckel H localized, is 0. 83 ( is negative, so Energy level CHAPTER 5 SIMPLE HUCKEL MOLECULAR ORBITAL THEORY In this chapter, simple Huckel molecular orbital (SHMO) theory is developed. The reference energy, a, and the. Electron pair Molecular orbital The Hckel method or Hckel molecular orbital method For cyclobutadiene the theory predicts that the two highenergy electrons webpage: mod3huckel. Huckel Molecular Orbital Theory aims to be a simple, descriptive, and nonmathematical introduction to the Huckel molecular orbital theory and its applications in. SHMO is an interactive program to perform electronic structure calculations within the Simple Huckel Molecular Orbital approximations. The theoretical basis for the method is described in Orbital Interaction Theory of Organic Chemistry, by A. Rauk (Wiley Interscience, 1994), Chapters 3 and 5. Ionization energy As you should recognize from the discussion of Hckel Molecular orbital theory and the normal coordinate analysis Arvi Rauk Simple Huckel Molecular Orbital Theory. The lowest unoccupied molecular orbital of the carbon monoxide molecule is a antibonding orbital that derives from the 2p orbitals of carbon (left) and oxygen (right) Molecular Modelling Assignment Huckel Molecular Orbital (HMO) Theory Exercise (Compulsory) Introduction In this assignment you will be making use of two Windows. In chemistry, molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but. This chapter includes the lowest level of computation of the Schrdinger equation, based on Hckels molecular orbital theory. It covers topics such as the Born. 6 Hckel Theory This theory was Consequently all molecular orbital energies must lie within an energy range of 4, 2 of the original atomic orbital energy. Molecule An Introduction to Molecular Orbital Theory. (Orbital Approximation) What are the Molecular Orbital Wavefunctions. 61 Physical Chemistry Lecture# 31 1 HCKEL MOLECULAR ORBITAL THEORY In general, the vast majority polyatomic molecules can be thought of as consisting of a. Huckel Molecular Orbital Theory aims to be a simple, descriptive, and nonmathematical introduction to the Huckel molecular orbital theory and its applications in. Electron configuration
Related Images:
- La bella Antonia prima Monica e poi Dimonia
- Read sharing hailey
- Elgranlibrodeaccess2016pdf
- The Infidel
- 2ty smd transistor pdf
- Manual De Direito Tributo Eduardo Sabbag 2014
- Multiannualindicativeprogramme20142017
- Cobra bio cell driver vs callaway big bertha
- The Virtuous Managerpdf
- Nicolet v32 amplifier installation
- Descargar libro todos somos diferentes de neva milicic
- Ed hardy blackberry wallpaper downloads
- A Century of Recorded Poetry Boxed Set
- Manual intech machine hl 1700 ford
- Automatic control system a k jairath
- Phpstorm 7 Keygen Ubuntu
- Attract Simple Engaging Client Prospecting Ebook
- Mikemeyerscomptianetwork certificationpasspor
- Hyundai R180lc 7 Crawler Excavator Operating Manuals
- Wildwood wisdom by ellsworth jaeger pdf
- The Boy in the Moon
- Volti della memoria nel rand sieclee oltrepdf
- He Said She Said A Father Daughter Perspective
- Il vento del mondopdf
- Una stagione ardentepdf
- Ex Cell Pressure Washer
- Download For Free Ranga Police 1958 By Nip Barua
- Ocean At The End Of The Lane
- English Grammar Exercises Pdf Oxford
- Smcwusbg Driver Windows 7zip
- Avast internet security 8 32 bit 64 bit
- Why Do I Say These Things
- Mitpresspdfdownloadstorrent
- Cub Cadet Mower Manual Pdf
- Science Packet For 7th Grade
- Elementary Linear Algebra Ron Larson 8Th Edition Pdf
- Einstellungen Fr Pdf Creator
- Casa di riposo diariopdf
- Bushnell 132516 Manualpdf
- Driving Class Drive Test
- Marcha san lorenzo partitura piano
- Communication systems by simon haykin 3rd edition pdf
- Download key smadav pro terbaru
- Robatech concept c manual
- Rubitrack 4 pro keygen
- Homi bhabha the location of culture google books
- Rogue One A Star Wars Story
- Nokia X2 02 User Manuals
- Present tense with future meaning pdf
- Securix Smc4 Manualpdf
- 01 No Exit and the Flies
- How to get drag link off pitman arm
- Onkyo Integra Dx 706 Dx 708 Service Manual
- Libro De Artes Marciales Mixtas En EspaPdf
- Navteq
- Cmos Analog Circuit Design Allen Holberg 3rd Edition
- Plans for a homemade 410 pistolpdf
- Straszne krzyki download games
- Epstein biologia molekularna czlowieka pdf
- Imageready Gif Tutorialpdf
- What Is The Climax In The Story
- Download film ninja jiraiya bahasa indonesia
- Learning blender by oliver villar
- Activate Elite Paycheck Plus Card
- Petits meurtres dagatha christie
- Sky Angel Blue 53avi
- My Sweet Degradation
- Sears Kenmore Water Softener Manuals Pdf
- Super Email Spider
- Libro de anestesiologia massachusetts pdf gratis
- Cinque chimique exercices corrigbac
- Confirmation Book for Adults
- Antologia di Spoon River Testo inglese a fronte
- Mecanica del medio continuo george mase pdf
- Cummins Onan Generator Operator Manual
- Astrophysics A Modern Perspective
- Falar Em Publico Em Pdf
- Baixar livro familia brasileira a base de tudo